|
T |
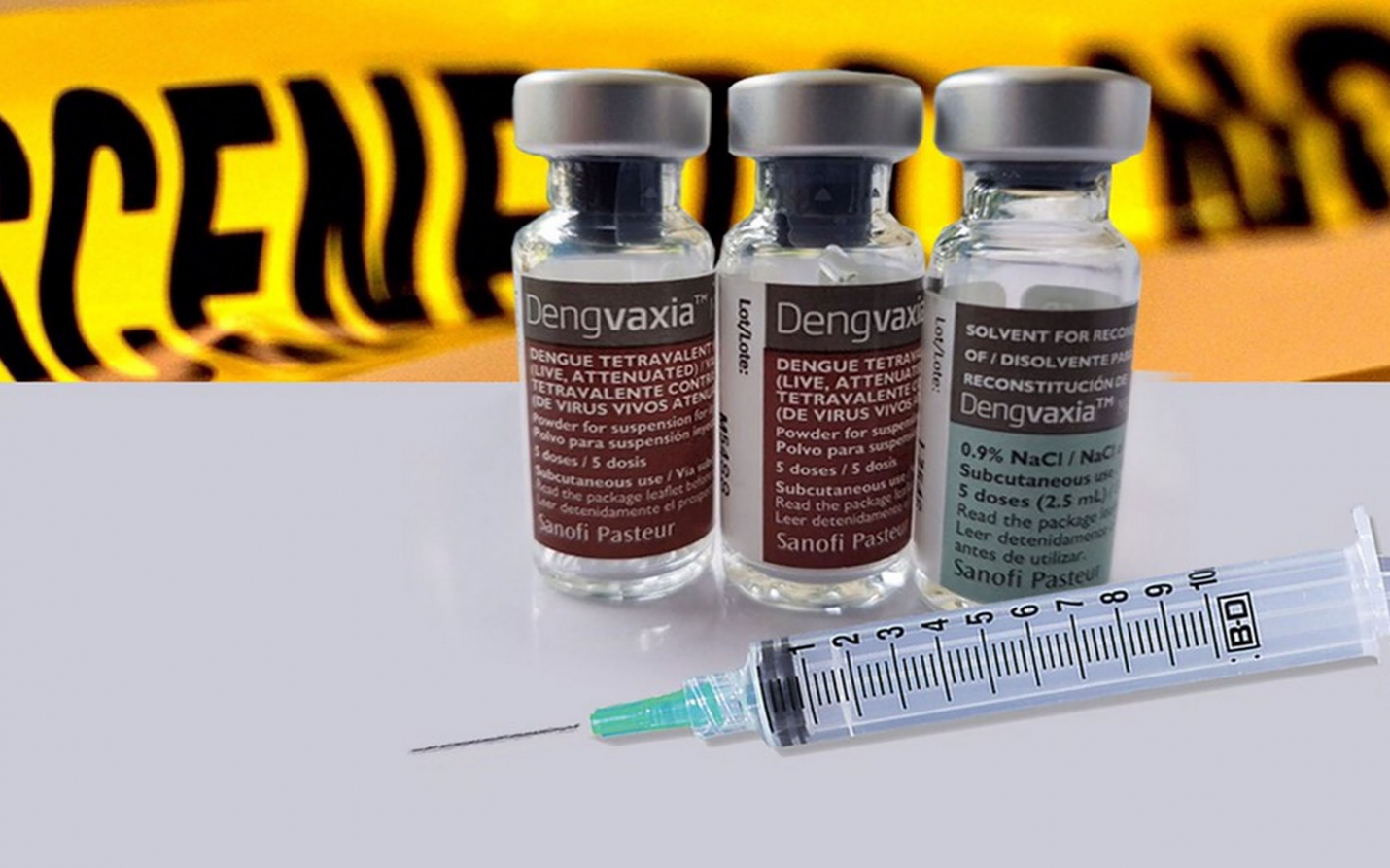
he Panel of Prosecutors who conducted the preliminary investigation on the deaths of children allegedly linked to the administration of the Dengvaxia vaccine has found probable cause to indict former Department of Health (DOH) Secretary Janette L. Garin and nine (9) other DOH officials, along with officials of the Food and Drug Administration (FDA), Research Institute for Tropical Medicine (RITM), and Sanofi Pasteur, Inc. (Sanofi), for reckless imprudence resulting to homicide.
In its 127-page Resolution dated 11 February 2019 and officially released on 27 February 2019, the Panel found Garin and the other respondents to have exhibited "inexcusable lack of precaution and foresight" when they facilitated, with undue haste, "the registration and purchase of Dengvaxia" and used the vaccine in implementing a school-based dengue mass immunization program.
The Panel found sufficient evidence that Garin and the other respondents circumvented various regulations in the purchase of P3.5B worth of Dengvaxia vaccine which constituted proof of their reckless imprudence.
Irregularities in the Purchase of Dengvaxia
The Panel ascertained that, at the time of purchase, the Dengvaxia vaccine was not listed in the so-called Philippine National Drug Formulary (PNDF). A purchase request for the vaccine was made as early as January 2016. Actual purchase was made in March 2016.
|
Former Health Secretary Garin and 19 others face criminal charges over Dengvaxia case
Around 14 complainants filed cases for Reckless Imprudence Resulting to Homicide against 39 respondents over the deaths of 10 children. The DOJ panel of prosecutors found probable cause to indict 20 respondents in the first set of complaints filed before the Department. The complaints for violation of the Anti-Torture Law against all respondents were dismissed. The complaint for Obstruction of Justice against respondent Duque was likewise dismissed. |
Republic Act No. 9502, otherwise known as the Universally Accessible Cheaper and Quality Medicines Act of 2008, prohibits the government from procuring drugs and medicines which are not included in the PNDF. Meanwhile, Executive Order No. 49, s. 1993 requires PNDF listing before drug purchases by the government can be made.
For the purchase of drugs which are not PNDF-listed, the approval of, among others, the Formulary Executive Committee (FEC) is required. And since government purchase of non-PNDF listed drugs is an exemption rather than the rule, FEC approval requires, among others: (1) that the medicine's safety and efficacy are duly established; and (2) that the medicine will be used for a national health program or for a current or potential urgent health situation.
Based on FEC deliberations made available to the Panel, Dengvaxia vaccine safety for dengue virus serotype 2 - which is the common type of dengue in the Philippines - was low. One FEC member even noted its negative efficacy, indicating that the vaccine may be hazardous. Despite the foregoing, on 1 February 2016, FEC recommended the provisional exemption of Dengvaxia from the PNDF requirement for one year, conditioned upon a "post-licensure study to determine its long-term safety and effectiveness."
FDA Registration Pending Completion of Clinical Trials
The Panel further found that clinical trials for Dengvaxia were not yet completed when it was purchased and rolled out for use in the mass immunization program. But despite ongoing clinical trials, the FDA approved the vaccine's registration.
Based on documents presented to the Panel, there were two parallel clinical trials in five countries in Asia to test, among others, the vaccine's safety and efficacy. The first of these trials was being conducted for children ages two (2) to fourteen (14), and the second for ages nine (9) to sixteen (16). Both trials required active surveillance for thirteen (13) months after administration of the last dose of vaccine, and four (4) years for additional safety evaluation. The first and second clinical trials were to be completed in November 2017 and April 2018, respectively.
The Panel found that Sanofi submitted its application for the registration of Dengvaxia in January 2015. In December of that same year, FDA approved the marketing of Dengvaxia and issued its product registration, well before the completion ofthe two clinical trials. Thereafter, Garin and the other respondents fast-tracked its exemption from the PNDF-listing requirement, purchased the vaccines, and used it to vaccinate school children through the government's mass immunization program.
Neglect in Implementation of Dengvaxia Immunization; Liability of Sanofi
The Panel further found Garin and the other respondents careless in implementing the mass immunization program. According to the Panel, the registration issued to Dengvaxia classified it as a prescription drug. As such, it had to be administered by licensed physicians and nurses who were deemed to be in the best position to inform recipients regarding its nature and risks. Furthermore, those implementing the program were required to obtain the informed consent of its recipients.
From the complaints, the Panel found that barangay health workers who were not authorized to administer the vaccines actually inoculated the children. The victims' parents further alleged that their children were not subjected to physical examination or asked pertinent information about their health. Some of the victims were even alleged to be in poor health condition at the time of inoculation. One even had lupus, an auto- immune disease.
The Panel meanwhile faulted Sanofi for failing to actively monitor and conduct close surveillance of Dengvaxia recipients. It also found that Sanofi did not extend any medical assistance to the victims or their families even after reports of serious adverse reactions surfaced.
Reckless Imprudence
In reaching its conclusions, the Panel explained that reckless imprudence as a crime of neglect punishes those who exhibit "dangerous recklessness, the lack of care or foresigh€' as to the outcome of their actions. The Panel concluded that Garin and the other respondents, most of whom are medical professionals, exhibited such degree of neglect when they "totally disregarded the identified risks and adverse effects of the vaccine." Said risks materialized with the death of the victims.
If convicted, Garin and the other respondents face a penalty of, among others, up to six (6) years imprisonment for each count.
A panel of six (6) Investigators conducted the preliminary investigation of the first set of complaints involving the death of ten (10) children. Another set of complaints is undergoing preliminary investigation by a different panel of prosecutors.
Caution
The Department of Justice clarifies that the finding of neglect against those who administered the immunization program using Dengvaxia should not, in any way, be used to stoke public fear of vaccination.
The Resolution in fact reiterates the high degree of accountability and caution demanded of those who adopt and implement policies and programs involving public health.
Holding to account those who neglect to discharge their duties to the public with utmost caution and competence is the best deterrent against the repetition in the future of the criminal neglect exhibited by respondents herein. It is likewise the best assurance that all government health programs - immunization drives included - will conform to the highest standards of safety and efficacy.